It may share electrons with an adjoining atom to make a covalent bond, or it might take one electron away to kind an ionic bond. As a outcome, halogens are probably the most reactive nonmetals, as they solely require one electron to type bonds. To create a covalent hyperlink, they both take away an electron from one other atom or share an electron from another storm. Because the valence electrons are at progressively higher energies in groups, the nonmetal’s reactivity reduces as a outcome of the atoms are unable to realize stability by acquiring electrons. The internal transition metals are the weather in the two rows which might be most often proven below the remainder of the opposite parts in the periodic desk. It is most secure to remember that the inner transition metals have 3 valence electrons, but as is often the case, there are a few exceptions to that rule.
The electron configurations for the primary 20 parts are proven here. I used foam circles I bought at a craft retailer for the electron markers. I even have also used candy prior to now, but found I usually needed to replenish the electron supply through the exercise. D- block components have 1-10 electrons within the d- shell.
Antraniks Smart Core Program
The electrons revolving within the outer most orbit of a atom has highest vitality level. Valence electrons are loosely hooked up to the nucleus. Therefore, less amount of power is required to drag an electron from the outer most orbits.
- As the oxidation state of an atom turns into bigger, so does its ability to draw electrons in a bond towards itself.
- These elements simply accept an electron to complete its valence shell.
- Browse other questions tagged electronic-configuration transition-metals or ask your own query.
- The most popularly recognized electrical conductor is copper, which has just one valence electron.
So in this sense the $1s$ electrons remain localised to a single atom while the $5p$ electrons are delocalised over both atoms. How can you establish the number of valence electrons from an electron configuration? Remember that our noble gases are throwing a party for every atom and the only requirement is having the proper amount of electron invitations.
In the case of HCl, the Cl has higher electronegativity. In the case of H2O, the O has greater electronegativity. We started with 23 electrons however in the diagram we now have 24. We’ve added an electron to complete it for the octet rule and due to that additional electron, that’s the reason it is -1.
Because valence electrons are on the outside of atoms they’re able to work together with different atoms. Have college students add information to the back of their tables like polyatomic ions, equations, key concepts, exceptions to guidelines. It helps them be taught it higher than reading it off one other chart and it provides cuantos electrones de valencia tiene el carbono them possession so that they hold them longer. Students should be in a position to group components together based on the number of valence electrons they contain. No free electrons which take part in chemical reactions .
Valence Electrons Of Inside Transition Metals
Both valency and valence electrons are applied for any chemical component. This is a color-coded desk made up of many alternative squares that lists all of the chemical components known to humankind. You can normally find these inside the cover of chemistry textbooks. There is also an excellent interactive table obtainable online here. The periodic table is split into four blocks by electron configuration.
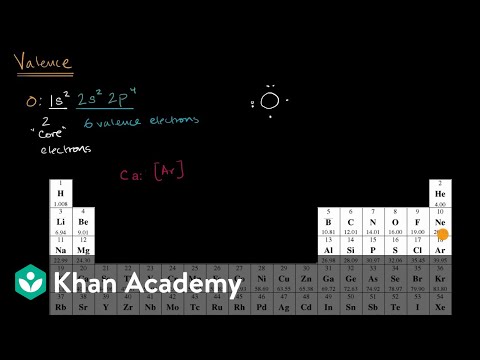
Losing two electrons is more difficult than losing one. As a result, they are less reactive, and these metals are more durable than group 1 elements. Four of the valence electrons are in lone pairs, which means that to find a way to obtain an octet configuration, the oxygen atom should interact in two single bonds or one double bond. By locating the element on the periodic desk, the table is sectioned off into s,p,d, and f blocks. Whichever portion of the desk the component falls beneath will present its valence electron sub-shell. By figuring out which interval the component is positioned inside determines the vitality stage of the valence.
It solely seems when there are double or triple bonds asymmetrically positioned. We also can find the valency of silicon with the assistance of the periodic desk. As silicon belongs to group 14 along with carbon , germanium , tin , lead , and flerovium . You must know how to find the number of valence electrons in a molecule so as to sucessfully draw Lewis Structures. Using the concept of proton-electron attractions students will see trends of atomic radius across rows of the periodic desk. Do not need to achieve or lose electrons to attain an octet.
The final shell of an element has 1, 2 or three electrons, those elements are referred to as metals. In chemistry, valence electrons are the electrons that are positioned in the outermost electron shell of an element. This is not true, because the transition metals can simply lose electrons from their d orbitals as well as the outer s orbitals. This query cannot be answered with out contemplating the orbital digital configurations of the weather. Noble gases have full orbitals and are, thus, nonreactive, giving them the identical traits as core electrons – low energy and a scarcity of a need to bond. Therefore, we are able to define all elements’ core electrons according to their earlier noble gas.
Be positive to know when to add or subtract from the last orbital for finding valence electrons. Noble gases have eight valence electrons – essentially the most steady state for a component. When the variety of electrons in an atom’s outermost shell approaches its most capacity, valency is set in a unique way. The outermost shell of the fluorine atom possesses seven electrons, and its valency might be seven, however it’s easier for it to gain one electron than to lose seven.